Abstract
Background: In the AML1310 trial (NCT01452646), a risk assessment based on the integration of cytogenetic/genetic and measurable residual disease (MRD) data was applied, to optimize patients' post-complete remission (CR) therapeutic allocation. By doing so and using the NCCN2009 risk-stratification, favorable-risk (FR) patients (NPM1mut/FLT3-ITDwt or CBF+ without c-Kit mutations) were to receive autologous stem cell transplant (AuSCT); poor-risk (PR) patients (adverse karyotype or FLT3-ITDmut) were to receive an allogeneic stem cell transplant (ASCT); intermediate-risk (IR) patients (intermediate karyotype or FLT3-TKDmut or CBF+ with c-Kitmut) were to receive AuSCT or ASCT depending on MRD levels, measured by flow after consolidation therapy.
With a median follow-up of 6 years, probability of 6-years OS and DFS of the whole series were 43.6% and 43.1%; cumulative incidence of relapse was 39.7%. Probability of 6-years OS and DFS were 58.5% and 50.1% in the FR category, 35.4% and 38.0% in the PR category, 43.1% and 45.7% in the IR category (Venditti, ASH2021).
Though time-to-event outcomes - including competitive events - represent the typical endpoints of clinical trials, this kind of analysis considers only terminal events. To this respect, multi-state models allow to analyze complex clinical trials where the final patients' outcome is influenced by intermediate events and to evaluate transition probabilities. In the AML1310 setting, we aimed at estimating the probability of i) being in an intermediate state (i.e. CR, transplant and relapse), ii) the effect of being in a transient state on the absorbing state.
Methods: We re-analyzed the AML1310 cohort (N=500) using multi-state models. By this approach we evaluated the effects of post-remission therapy (ASCT or AuSCT) on relapse and death in patients achieving CR. CR, Relapse, Transplant, Relapse after transplant are denoted as intermediate states where a patient can go through and then many events may occur. No relapse mortality (NRM), Relapse mortality (RM), NRM post-transplant, RM post-transplant are denoted as absorbing state meaning no further transitions are possible once a patient enters any of these states. We developed time in homogeneous Markov multi-state models meaning that the hazard of transition from one state to another does not depend on the time spent in the current state, but only on the current state and the time since treatment initiation.
Being the AML1310 a risk-adapted and MRD-oriented trial, we investigated possible subgroup effects based on NCCN2009 risk classification. For this purpose, we estimated the main multi-state model in each subgroup.
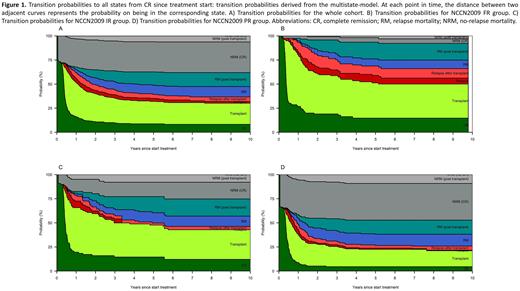
Results: In the whole cohort, the probability at 6 years of no relapse mortality (NRM) without transplant was 31.7%; while the NRM after transplant was 6.32%. The probability of being alive without relapse at 6 years was 30.8%: including 22.6%% after transplant and 8.2% without transplant (Fig. 1A). Next, the same analysis was performed stratifying the population according the NCCN2009 risk. As depicted in Figure 1, the probability of being in Relapse (Relapse + Relapse post-transplant + RM + RM post-transplant) was greater in the FR subgroup (Fig. 1B) in comparison to IR (Fig. 1C) and PR (Fig. 1D, 42.2% vs 31.9% vs 30.3%). This difference is due to a greater probability of being alive, most likely because of the use of ASCT in second line. In the IR subset (Fig. 1C), the probability of NRM in CR was lower than in the PR patients (25.2% vs 47.2%). In addition, the risk of being alive at 6 years after transplant was comparable between IR and FR groups (30.7% vs 35.3%) and significantly greater than the PR (17.9%). These findings are attributable to the efficacy of the MRD-oriented strategy applied to the IR group.
Conclusion: By using a multi-state model, we observed that, in the AML1310 setting, FR patients experienced a high risk of relapse with an inferior mortality rate after relapse, thus suggesting the need of an MRD-driven transplant decision also for this subset. Furthermore, the use of an MRD-driven approach in the IR group translated into a better NRM and survival after transplant, corroborating the goodness of the strategy. These results enforce the earlier reported beneficial effect of the risk adapted strategy in AML and show that multi-state models deepen the knowledge of the effect of treatment strategy on competing and series of events.
Disclosures
Del Principe:Jansenn: Honoraria; Gilead Sciences: Honoraria; Incyte: Honoraria. Venditti:abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen & Cylag: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Medac: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Speakers Bureau; astrazeneca: Honoraria; Amgen: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal